To further understand Thermodynamics Processes, Watch this video:
During the volume change, the pressure and temperature may also change.To evaluate the equation above, we would need to know how pressure varies with volume for the actual process by which the system changes from state i to state f.
Pressure-Volume
Diagrams (PV diagrams) are useful tools for visualizing the thermodynamic
processes of gases. These diagrams show pressure on the y-axis, and volume on
the x-axis, and are used to describe the changes undergone by a set amount of
gas. Because the amount of gas remains constant, a PV diagram not only tells
you pressure and volume, but can also be used to determine the temperature of a
gas when combined with the ideal gas law.
(a) The shaded area represents the work W done by a
system as it goes from an initial state to a final state f. Work Wis
positive because the system’s volume increases. (b) W
is still positive, but now greater. (c) W is still
positive, but now smaller. (d) W can be even smaller
(path icdf ) or larger (path ighf). (e) Here the system
goes from state f to state i as the gas is compressed to
less volume by an external force.The work W done by the system
is now negative. (f) The net work W net done by the
system during a complete cycle is represented by the shaded area.
Example:
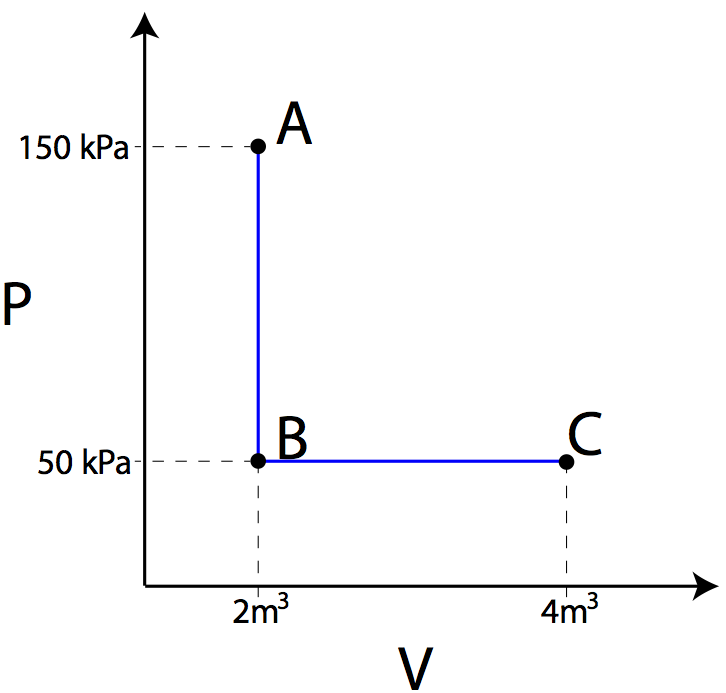
1.Using the PV diagram at right, find
the amount of work required to transition from state A to B, and then the
amount of work required to transition from state B to state C.
First law of Thermodynamics
The First Law of Thermodynamics states that heat is a form of energy, and thermodynamic processes are therefore subject to the principle of conservation of energy. This means that heat energy cannot be created or destroyed. It can, however, be transferred from one location to another and converted to and from other forms of energy. Internal energy of the material depends only on the material’s state (Heat, temperature, pressure, and volume). Heat is the energy exchanged between the system and its surroundings. Hear is positive if the system absorbs heat and negative if the system loses heat. Work done by the system is positive if the system expands against an external force from the surroundings and negative if the system contracts because of an external force.
Example:
1.Five thousand joules of heat is added to a closed system, which then does 3000 joules of work. What is the net change in the internal energy of the system?
Some Special Cases of the First Law of Thermodynamics
1. Adiabatic processes. An adiabatic process is one that occurs so rapidly or occurs in a system that is so well insulated that no transfer of energy as heat occurs between the system and its environment.Putting Q=0 in the first law yields:
2. Isochoric or Constant-volume processes. If the volume of a system (such as a gas) is held constant, that system can do no work. Putting W= 0 in the first law yields:
3. Isobaric processes. It is a process which occurs at constant pressure. When the system is heated the volume goes up and piston moves upwards so the system does work and when the piston goes down you do work to the system making the volume goes down.
4. Isothermal processes- This process occurs at a constant temperature, this typically
occurs when a system is in contact with an outside thermal reservoir, and the
change occurs slowly enough to allow the system to continually adjust to the
temperature of the reservoir through heat exchange.
5. Cyclical processes. There are processes in which, after certain interchanges of heat and work, the system is restored to its initial state. In that case, no intrinsic property of the system—including its internal energy—can possibly change. Putting ΔEint = 0 in the first law yields:6. Free expansions. These are adiabatic processes in which no transfer of heat occurs between the system and its environment and no work is done on or by the system. Thus,Q = W = 0, and the first law requires that:
To learn more about Thermodynamics watch these videos:
Reference:
David Halliday, Jearl Walker, and Robert Resnick, Fundamentals of Physics 10th edition
https://www.youtube.com/watch?v=dHdlH3l8FkM
https://www.youtube.com/watch?v=uyEwWZVSIrA
https://aplusphysics.com/courses/honors/thermo/thermodynamics.html
https://www.youtube.com/watch?v=4i1MUWJoI0U

















i rate 8/10 informative though some topics are missing.��
ReplyDelete